Turkey begins clinical trials for Chinese virus vaccine
ANKARA

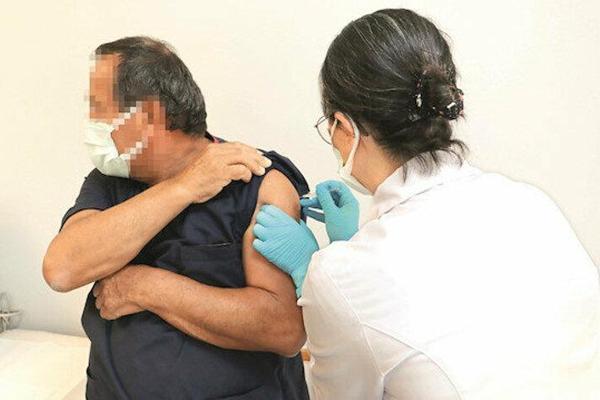
Turkey has started testing the Chinese Sinovac vaccine for COVID-19 on three volunteers in its first clinical trial, the country’s health minister has said.
Fahrettin Koca said the vaccine trials will be held in two phases: On 1,200 volunteers in the first and 10,000 in the second.
Health workers will volunteer for the trial in droves, he added, speaking after the Health Ministry’s Science Board meeting on Sept. 16.
The minister reminded that Turkey already gave permission for test trials for a vaccine which is being developed by Pfizer.
“Test trials for Pfizer’s potential vaccine are expected to begin at more than 10 institutions in the coming days,” Koca said.
He noted that Russia had already applied for test trials in Turkey for its own vaccine.
“What we understand is that the tests are production positive results. We may give permission for the third phase test trial for the Russian vaccine next week,” Koca said.
The scientific community is of the consensus that the vaccine studies will show positive results by the year end, according to Koca.
Asked if the Science Board is recommending curfews in the face of a spike in the number of COVID-19 cases, the minister responded that the world and Turkey are in a more difficult phase of COVID-19 than at the beginning of the pandemic and said that the nations in the world are avoiding such a measure and it is not a preferred curb globally.
“For the time being imposing lockdowns is out of question for Turkey and the Science Board has not made such recommendation,” Koca said.
He reiterated the importance of wearing masks, keeping social distancing, and ensuring proper hygiene to stem the spread of the coronavirus.
“Thus, the local health boards in provinces have made decisions to reduce mobility, such as flexible working hours and also announced measures for public transport. The Science Board may also make recommendations for flexible working hours for the private sector depending on the state of the outbreak in a particular province,” Koca said.
The minister also stressed 51.6 percent of the beds in hospitals were occupied, intensive care units are working at 66.3 percent capacity and 33.6 percent of the ventilators are being used.
Turkey also raised the number of filiation personnel to 11,238 from 6,000 to monitor the spread of the virus, according to Koca. The country’s filiation teams conduct contact tracing to find people potentially infected with the virus.
Professor Serhat Ünal from the Science Board and Professor Murat Akova at Hacettepe University, where the clinical tests were launched, on Sept. 17 provided further details on the trials.
“The number of centers where the tests will be carried out will gradually increase to 25 and a total of 13,000 people will take part in the trials. We have been preparing for this for three months,” said Ünal.
In the first phase, 1,200 health workers, who are most exposed to the risks, will be vaccinated, according to Akova.
Volunteers, who are aged between 18 and 60, will be divided into two groups, some will be administered the vaccine and some will be given a dose of placebo, Akova said, adding that the likely side effects are malaise and headache. “We are informing the volunteers about the side effects.”
Akova added that it is not known yet for how long the vaccine could protect people against COVID-19.
“A vaccine that could provide protection for four months is good enough for us as the contamination risk will increase when the weather gets cold. We are very worried about the months of October, November and December,” he said.
In the worst-case scenario, the vaccine’s protection is estimated to be three to four months or even up to six months, in the best-case scenario it is three to four years, according to Ünal.
“Even if it provides protection for six months, we will have the chance to take the pandemic under control,” he said.
